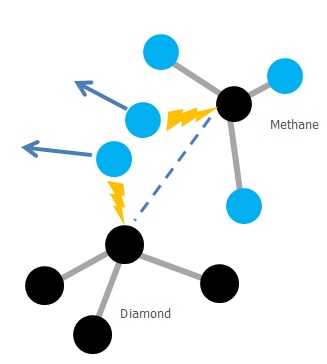
This is just a theoretical method based on these suppositions: - Diamonds and graphite differ only in their structure. - Heat and pressure is not essential criteria in turning graphite into diamond. - Diamond have a free bound at their outer layer which is commonly bounded with H atom - Hydrogen-carbon bound can be broken easily and replaced with another carbon atom
By using an existing diamond as seed, the diamond must be place in sealed chamber filled with Argon gas to prevent anything but carbon gas bounding with the diamond.
To knock out the Hydrogen at the outer layer and replace it with a carbon atom, it is subjected to a high flow of CH4 gas. The trick is to ensure the gas hits the atom at the right speed and energy to knock it out and replace it. If that is not considered or measured very accurately many attempt will fail leading to assumption that the process is flawed and is therefore abandoned.
The reason CH4 is used is that it contains a single Carbon atom and it is already surrounded by four Hydrogen atoms, making is balanced and therefore the bounding easier.
Using a high-power beam of ultraviolet light would excite the outer hydrogen layer and make them more susceptible to exchange of H to C.
Since heat and pressure is omitted from the process the processes will take time, but it will be cheaper.
This all can be calculated based on the probability of atoms hitting each other in the right way. In nature extreme heat and pressure increases this probability by billions, but this method can produce diamond if these factors are accurately controlled.